14+ orbital diagram ca
Figure 924 Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 8-10 The energy diagram we have just generated fits experimentally. The orbitals are 1s 2s 2p 3s 3p and 4s.
Internet Database Of Periodic Tables Chemogenesis
These orbitals are filled with electrons the amount of electrons depends on which.

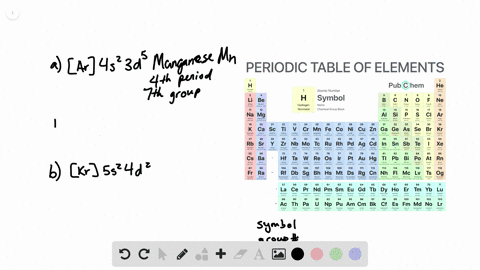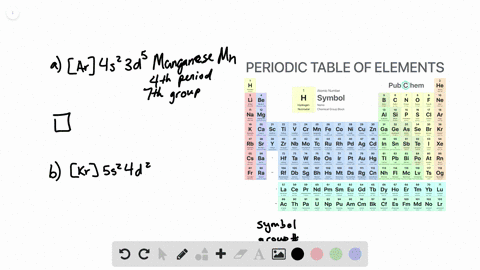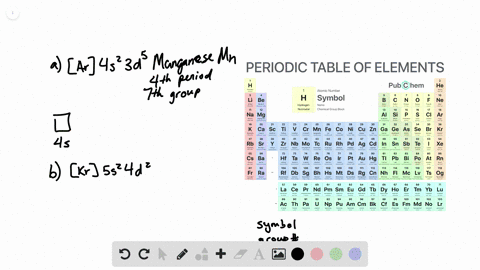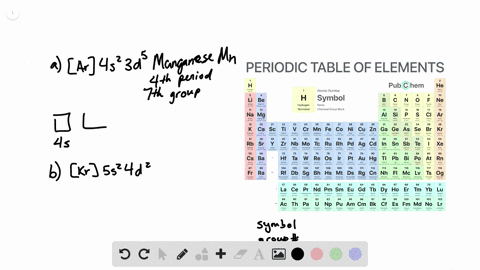
. General Chemistry Lecture Professor Gerard Parkin Fall 2019 chapter 14. To write the orbital diagram of chromium Cr you have to do the electron configuration of chromium. Interactive Periodic Table Let me tell you how this Interactive.
1s is the closest and lowest energy. Which has been discussed in detail above. Remember that s orbitals contain a maximum of two electrons p orbitals a maximum of six d a maximum of 10 and f a maximum of 14.
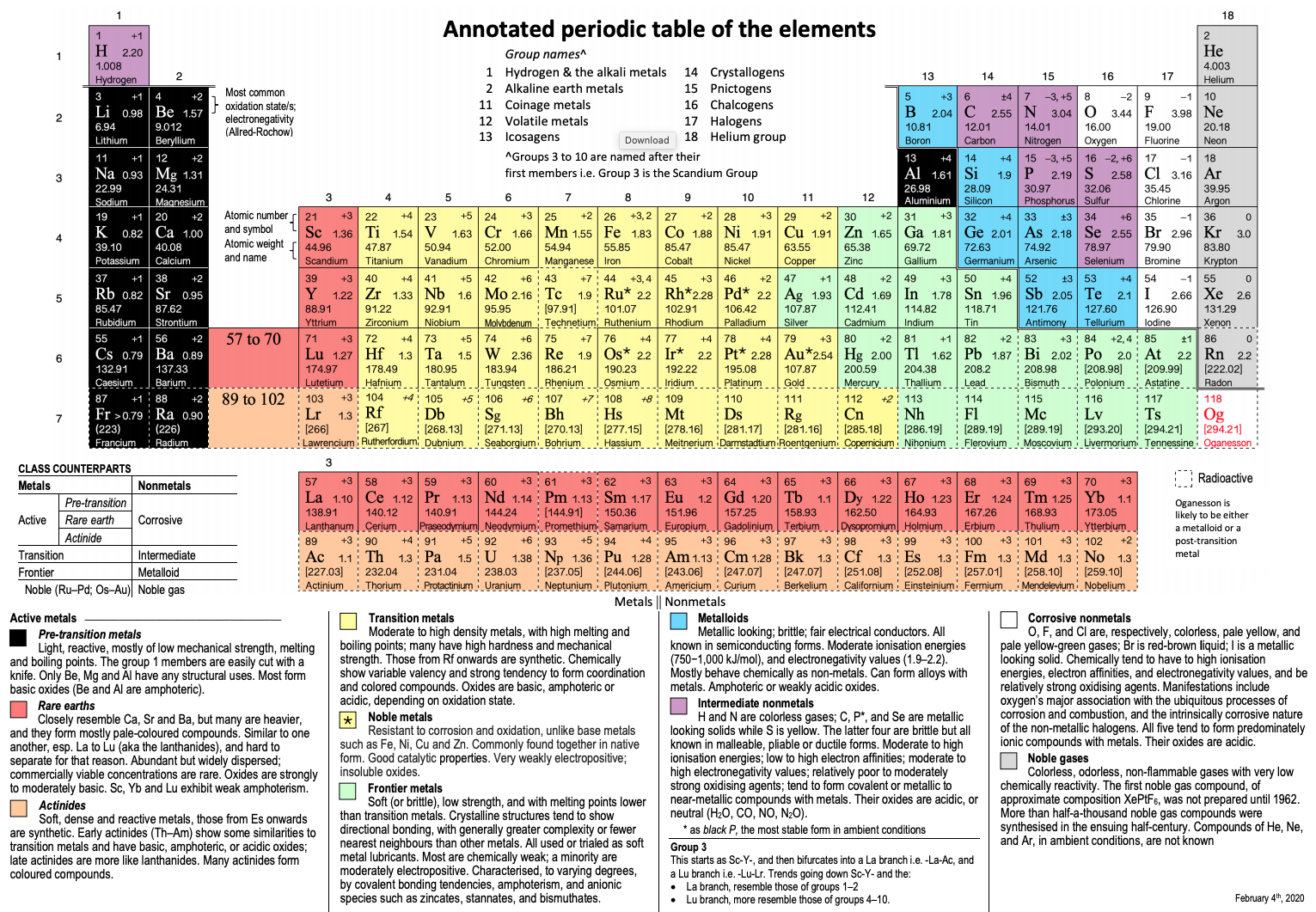
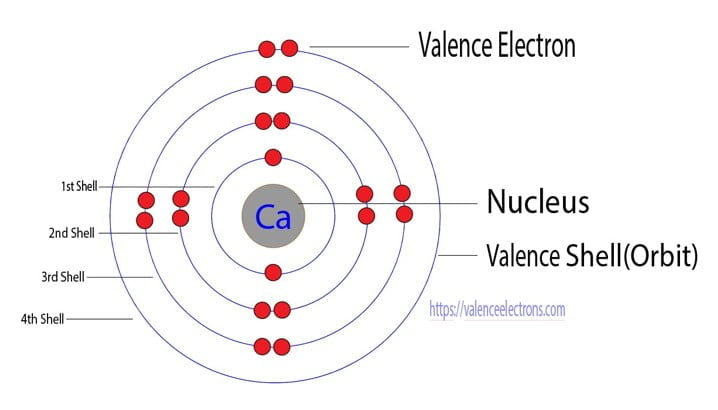
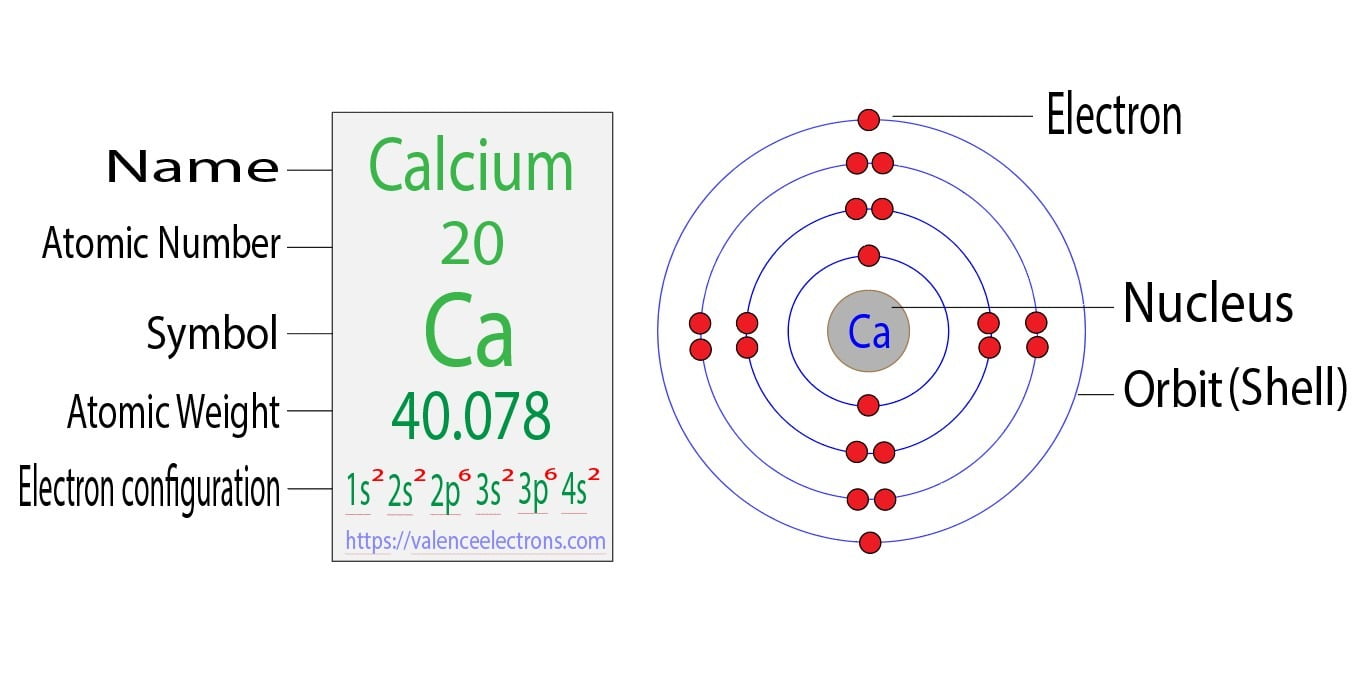
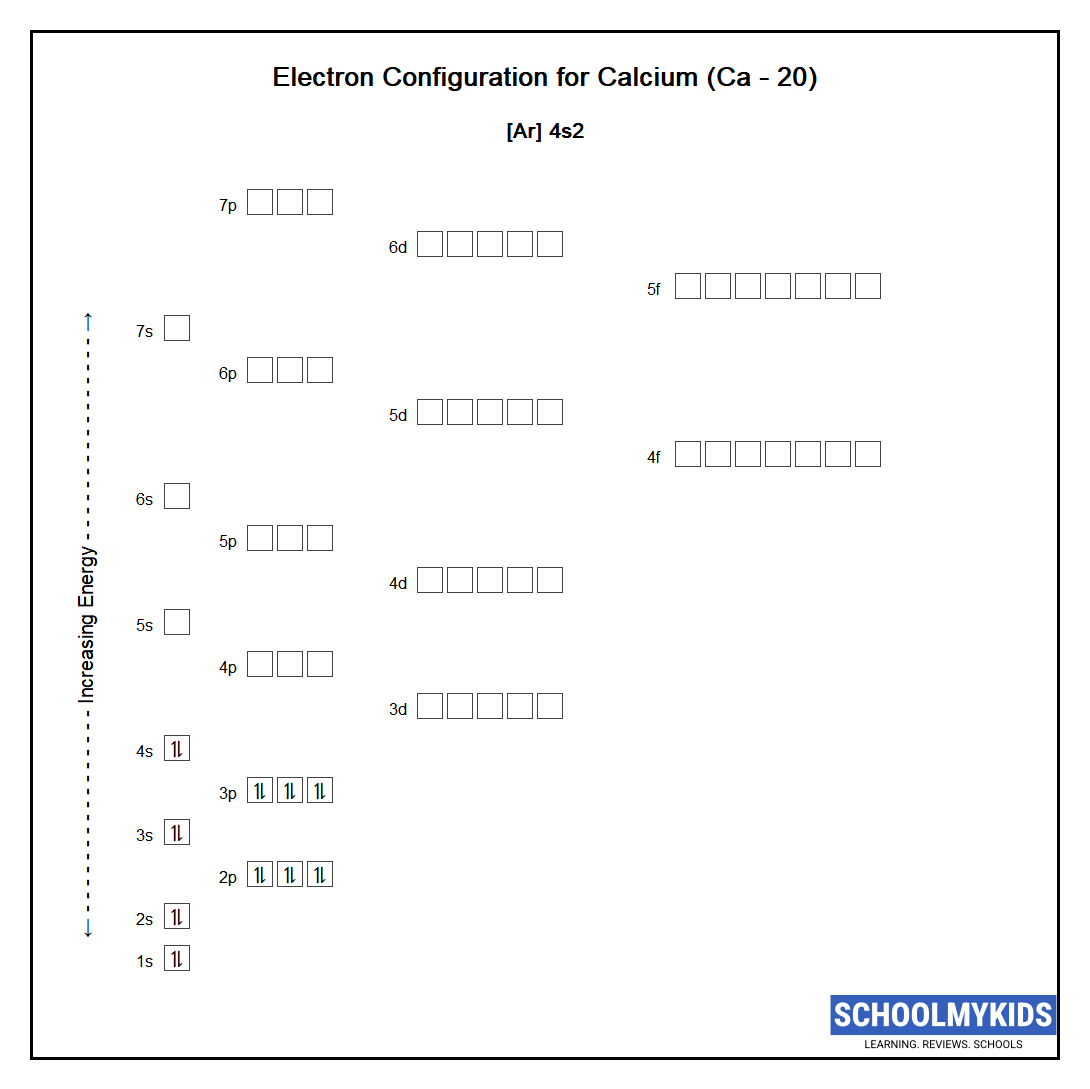
Orbital diagram for Calcium The orbital diagram simply represents the arrangement of electrons in the different orbitals of an atom it uses an arrow to represent the electrons every orbital. The Aufbau principle tells you. There are different types of orbitals that all have different energy levels.
Orbitals problems with lewis structure provides indication of the. The orbital diagram for Calcium is drawn with 6 orbitals. Finally an f orbital has a maximum of 14 electrons so seven connected boxeslines are used for any f orbital.
No because due to the Auf-bau rule e- enters the 4s sub-shell of Ca instead of going in 3d sub-shell so Ca does not have any vacant d-orbital. The orbital diagram simply represents the arrangement of electrons in the different orbitals of an atom it uses an arrow to represent the electrons every orbital one box contains a maximum. Free Gift for you.
The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. The ground-state electron configuration of cadmium is 1s 2 2s 2 2p 6. The Basics of Orbital Diagrams.
What is the electronic. Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. When filling the boxes of each orbital with arrows it is important to.
Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given.
Calcium Ca Electron Configuration And Orbital Diagram
Calcium Ca Electron Configuration And Orbital Diagram

Indium Iii In The Periodic Table Of Di 2 Pyridyl Ketone An Unprecedented Transformation Of The Ligand And Solid State 115in Nmr Spectroscopy As A Valuable Structural Tool Inorganic Chemistry
Principles Of Chem 1

Effect Of Strongly Coupled Plasma On The Magnetic Dipolar And Quadrupolar Transitions Of Two Electron Ions Physics Of Plasmas Vol 20 No 4

Anion Centered Polyhedron Strategy For Strengthening Photon Emission Induced By Electron Phonon Coupling Inorganic Chemistry
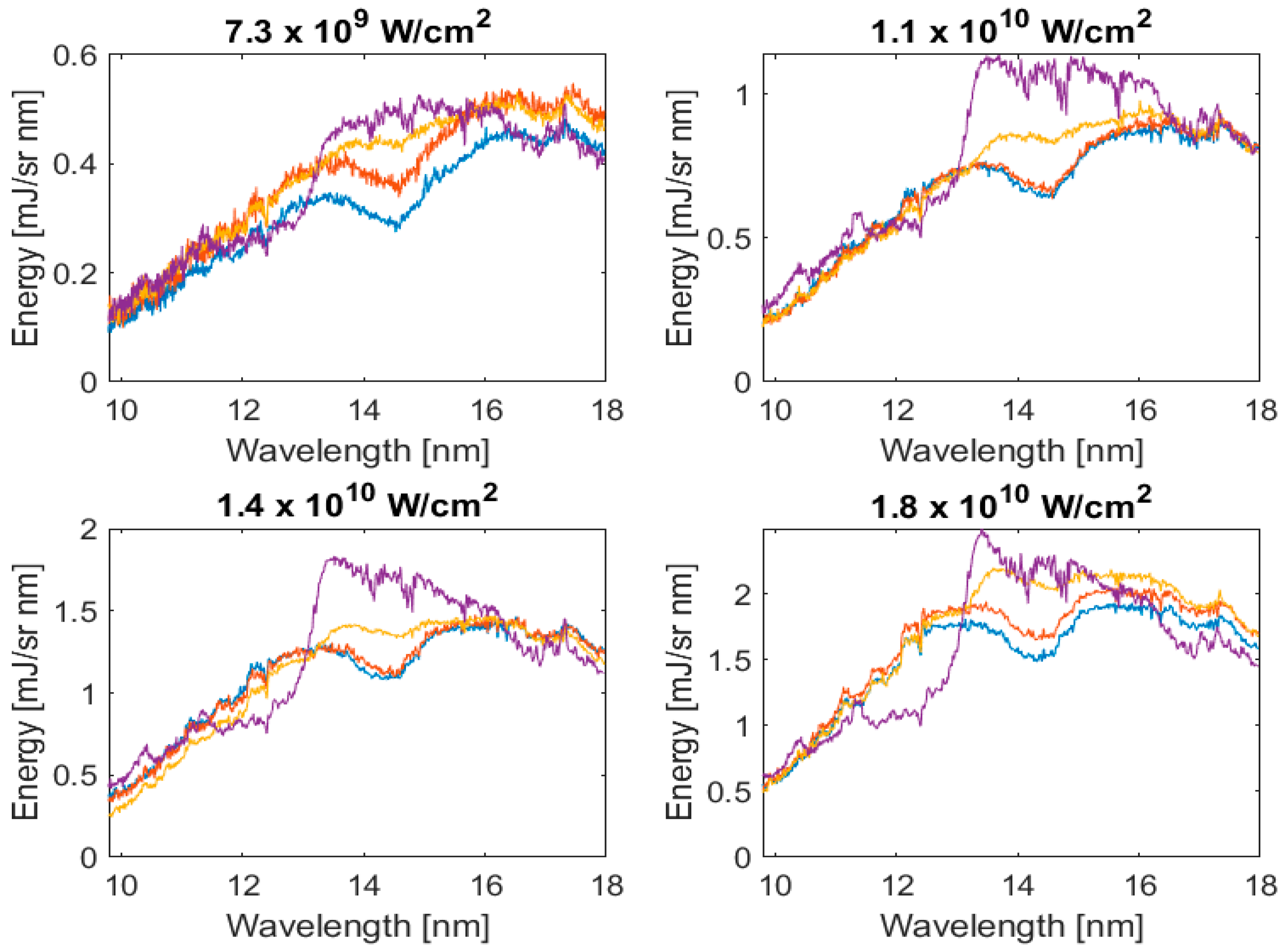
Atoms Free Full Text An Investigation Of Laser Produced Lead Tin Alloy Plasmas Between 10 And 18 Nm Html
Chapter 8 Electron Configuration And Chemical Periodicity Video Solutions Chemistry The Molecular Nature Of Matter And Change Numerade

Phase Diagram Of Q1d Cuprates Sr 14 X Ca X Cu 24 O 41 Tomislav Vuletic Zagreb Naslov Ppt Download

Draw The Orbital Diagram Ca 2 Ion And State The Number Of Three Fundamental Particles Present In It
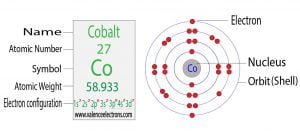
Calcium Ca Electron Configuration And Orbital Diagram

Synthesis Of Bimetallic Copper Rich Nanoclusters Encapsulating A Linear Palladium Dihydride Unit Chakrahari 2019 Angewandte Chemie International Edition Wiley Online Library

Metal Anions In Metal Rich Compounds And Polar Intermetallics Whangbo 2011 European Journal Of Inorganic Chemistry Wiley Online Library
Chapter 8 Electron Configuration And Chemical Periodicity Video Solutions Chemistry The Molecular Nature Of Matter And Change Numerade

What Is The Orbital Diagram For Calcium Homework Study Com
In Molecular Orbital Theory Why Do We Follow A Certain Electronic Configuration Upto Nitrogen And After That Electronic Configuration Is Changed Why Quora
Ca Calcium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids